Background: Elderly patients with relapsed/refractory (R/R) FL have limited treatment options. Lenalidomide has direct tumoricidal and antiangiogenetic actions on tumor cells and is able to modulate tumor-cell microenvironment. Lenalidomide combined with Rituximab (R 2) in induction has shown a good clinical activity with low toxicity in both untreated and R/R FL patients. R 2 as maintenance treatment is less studied.
Methods: RENOIR (NCT02390869) is an Italian FIL multicenter phase III open-label study for elderly subjects with grades 1-3a R/R FL in advanced stage who received 1 or 2 prior anti-lymphoma therapy and required treatment. Induction treatment consisted in 4 cycles of standard Rituximab-chemotherapy (Bendamustine or CHOP or CVP) according to physician choice or to previous lines. Two additional courses of R-chemotherapy were given for patients with partial remission (PR) or stable disease (SD). Those with stable disease (SD) or better after induction were randomized 1:1 to standard arm (ARM A) with Rituximab maintenance (Rituximab IV 375 mg/m 2 every 12 weeks for 8 doses) or to experimental arm (ARM B) with R 2 maintenance (Rituximab IV 375 mg/m 2 every 12 weeks for 8 doses and Lenalidomide 10 mg/day, days 1-21 per 28-day cycle for 24 cycles).
The primary endpoint was 2-yr progression-free survival (PFS) comparing R 2 vs standard Rituximab maintenance using a two-sided test with alpha=0.05. An improvement of PFS from 60% to 78% in favor of R 2 arm was considered relevant. Secondary endpoints included safety, overall survival (OS), response rates (ORR), complete remission (CR), minimal residual disease (MRD) and quality of life (QOL).
Results: From May 2014 to October 2022, 152 subjects were enrolled. Median age was 71 years (range, 67-77), 55% male, 82% had stage III/IV disease, 41% and 39% were at FLIPI intermediate-high or high risk, 25% had LDH above normal value, 78% relapsed and 22% were refractory to last treatment. One or 2 prior lines were given to 71% and 29%, R-Bendamustine was the preferred induction treatment (83%). All patients received prior Rituximab. At the end of induction ORR was 84% (n = 128) with CR 57% (n = 87). One hundred and twenty-eight (84%) completed the induction phase and 24 (16%) discontinued treatment because of: 9 progressive disease, 6 withdrawals of consent, 6 adverse events (AEs), 1 death, 2 other.
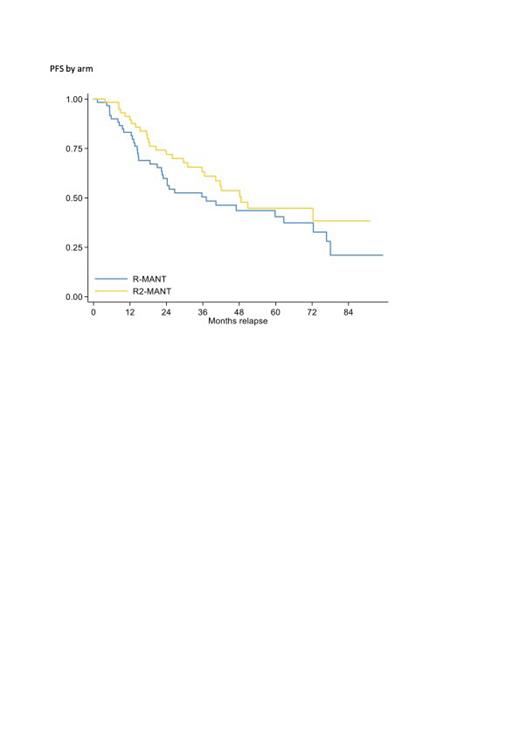
One hundred and twenty-eight patients were randomized (ARM A 64 and ARM B 64) with well-balanced clinical characteristics. During maintenance 34 patients in ARM A and 38 in ARM B discontinued the treatment or are ongoing. At a median follow up of 58 months, 2-yr PFS rate was higher in R 2 arm, though not statistically significant: ARM B vs ARM A 2-yr PFS 72% vs 60% (HR 0.69, 95%CI 0.42-1.14, p=0.149 Fig 1). Subgroup analysis suggests a greater benefit in terms of PFS of R 2 in patients <70y (R 2 vs. R HR=0.34 vs HR=1.00 p-interaction=0.066 for patients < 70 and >=70 respectively). Two-yr OS rates for ARM B vs ARM A were: 80% vs 89% (HR 0.91, 95%CI 0.48-1.73, p=0.777). At three years from the randomization, patients who required further treatment were: 14% in ARM B vs 28% in ARM A (HR 0.64, 95%CI 0.31-1.32, p=0.225).
The overall 2-yr PFS and OS rates from enrollment were: 66% (95%CI 57-73) and 83% (95%CI 75-89). The most common grade 3/4 AEs during maintenance in ARM A vs ARM B were: neutropenia (15% vs 41%, p=0.002), gastrointestinal disorders (2% vs 9%, p=0.116), infections (3% vs 11%, p=0.166). Deaths were 49: 10 patients not randomized (5 progressive disease, 1 COVID, 2 infections, 1 secondary neoplasia, 1 cachexia); 20 in ARM A (4 secondary neoplasia, 4 COVID, 4 other infections, 4 unknown, 2 progressive disease, 1 car accident, 1 cachexia) and 19 in arm B (3 secondary neoplasia, 5 COVID, 2 infections, 2 heart attack, 2 unknown, 3 progressive disease, 2 cachexia).
Conclusions: the addition of Lenalidomide to Rituximab as maintenance treatment had a clinical benefit but with a limited impact on the overall outcome in this cohort of elderly R/R FL. R 2 maintenance reduced the risk of progression mostly in patients < 70 years, but without a statistically significant difference and with a higher number of adverse events that led to R 2 interruption more frequently. Indeed, the overall outcome of this elderly R/R cohort of FL patients was excellent with a short chemoimmunotherapy followed by two years of Rituximab ± Lenalidomide. Different treatment strategies or a different R 2 schedule might improve the outcome.
OffLabel Disclosure:
Botto:Takeda: Speakers Bureau. Zilioli:Incyte: Speakers Bureau; Lilly: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Servier: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Other: travel expenses, Speakers Bureau; Roche: Consultancy, Other: travel expenses; MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cavallo:Roche: Honoraria, Speakers Bureau; Takeda: Research Funding; Astra Zeneca: Research Funding; Beigene: Research Funding. Merli:Gilead: Other: advisory board; Roche: Other: advisory board; Novartis: Other: advisory board; Takeda: Other: advisory board; Incyte: Other: advisory board; Janssen: Other: advisory board; MSD: Other: advisory board. Tani:Abbvie, Jansen-Cilag, Incyte: Membership on an entity's Board of Directors or advisory committees. Conconi:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gentili: Membership on an entity's Board of Directors or advisory committees, Other: travel fees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead/Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Corradini:Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Gaidano:Abbvie and Janssen: Speakers Bureau; Abbvie, Astra-Zeneca, BeiGene, Incyte, Janssen, Lilly: Other: Advisory board. Arcari:Janssen, Abbvie, Takeda, Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Musuraca:Janssen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Luminari:ROCHE: Membership on an entity's Board of Directors or advisory committees; Jannsen: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Regeneron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incute: Membership on an entity's Board of Directors or advisory committees. Ladetto:Novartis: Honoraria. Vitolo:Servier: Other: Lecture Fees; Roche: Other: Lecture Fees; Janssen: Other: Lecture Fees; Incyte: Other: Lecture Fees; AbbVie: Other: Lecture Fees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees.
Lenalidomide was off label at the moment of study begin and was supplied by Celgene